Cell culture is the cornerstone of biomedical research and biotechnology innovation. Although bioproduction of biomedicines in bioreactors is now the norm, and the use of organoids andorgan-on-a-chip (OoC) is growing, the beginnings of in vitro cell culture were more modest.
The discovery of cells
The cells, named by a scientist...
The history of cell culture begins with the discovery of the cells themselves, which is closely linked to the evolution of the microscope: it was the improved magnification provided by the lenses that enabled cells to be observed for the first time.
In 1665, Robert Hooke first used the term “cell “. Examining cork under a microscope, Hooke noticed that it was made up of small chambers, which he then called “cells” because they reminded him of the monks’ chambers in a monastery, cellula in Latin. However, Hooke observed only dead plant cell walls, not living cells.
... but discovered by a draper
It was not a scientist, but a Dutch cloth-maker, Antonie van Leeuwenhoek, who first observed living cells under the microscope. Fascinated by lens making and microscopy, which he used in particular to examine the quality of his fabrics, he perfected the polishing of lenses to obtain a magnification of 300 times. A feat for the time, since the multi-lens microscopes developed by Hooke only achieved 30 to 40 times magnification.
In 1674, Van Leeuwenhoek was the first to observe micro-organisms and cells with his ingenious microscopes. In a letter to London’s Royal Society, he described in detail what he observed: his first observations included bacteria, protozoa and blood cells. He called the small living beings he saw through his microscope “animacules”. So, despite his lack of scientific education, Antonie van Leeuwenhoek is considered by some to be the very first cell biologist.
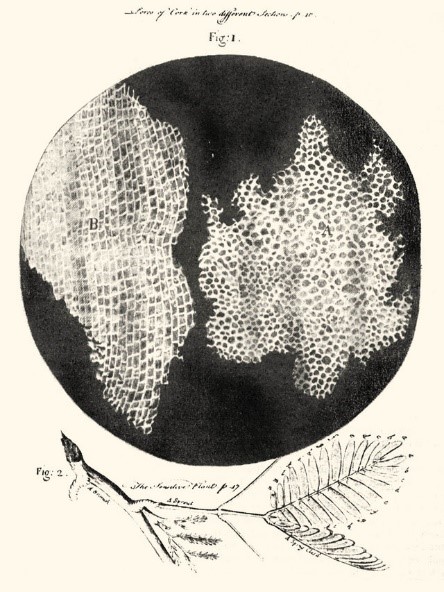
Robert Hooke, Micrographia (excerpt), 1665. Drawing of the cellular structure of cork and a strand of the sensitive plant

One of Antonie van Leeuwenhoek’s microscopes, late 17th century
These fundamental discoveries were the start of what would later become cell theory, which today forms the basis of our understanding of the structure of living organisms.
The beginnings of cell culture
Tissue culture pioneers
Following the discovery of cells, scientists progressively developed techniques to study them outside their original organism: the first experiments therefore aimed to understand how to maintain and observe living tissue in vitro.
One of the earliest known attempts was that of Wilhelm Roux, a German embryologist, who kept chicken spinal cord cells alive in saline solution for a few days. One of the first significant successes is attributed to Ross Harrison, an American zoologist, and his ” hanging drop ” technique, which involved placing tissue fragments in drops of coagulated lymph. Carried out in 1907, his work not only demonstrated that cells could survive and develop in vitro, but also that they could maintain specific interactions, such as the growth of neuronal axons.
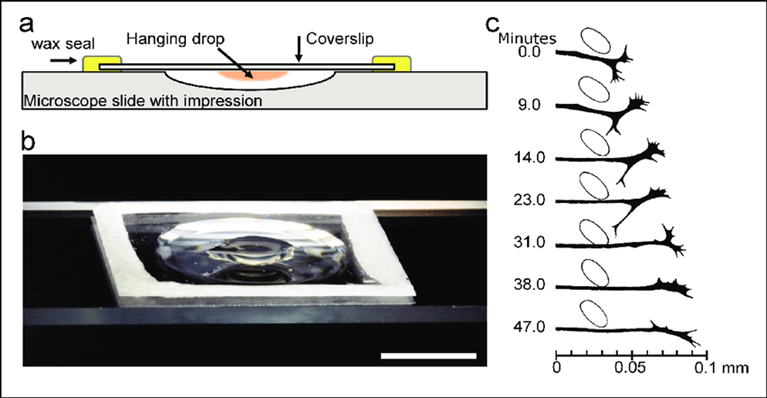
From Millet, Larry Gillette, Martha. (2012).
Over a Century of Neuron Culture: From the Hanging Drop to Microfluidic Devices.
Alexis Carrel, a French surgeon, and his assistant Montrose Burrows then adopted this technique, replacing lymph with plasma and adding a mixture of serum, saline solution and chick embryo extract. This mixture, which they called plasma medium, became standard until the 1950s. They thus laid the foundations of tissue culture, which they defined as ” a plasma medium seeded with small fragments of living tissue “.
Having successfully maintained cells in culture for 34 years, Carrel was firmly convinced that primary cells in culture were immortal if maintained in an appropriate nutrient medium. The definitive proof of this error was provided by Leonard Hayflick, who demonstrated in 1965 that normal human fibroblasts do not survive in vitro beyond around 50 divisions: what is now known as the “Hayflick limit”. The survival of Carrel’s culture was probably due to the addition of new cells from the embryonic extract contained in the medium.
Subsequently, these culture techniques develop, and primary cells can be harvested from a variety of sources, separated by trypsin and grown in monolayers with an appropriate serum-containing medium.
The HeLa cell revolution
In 1951, Henrietta Lacks, a 31-year-old African-American woman, went to Johns-Hopkins Hospital to be examined for a stomach ache. She was diagnosed with cervical cancer: an extremely fast-growing tumor.
Without her consent, Dr. George Otto Gey biopsied and cultured the carcinoma cells, expecting them to die after a few divisions, as most samples did. However, the cells labeled “HeLa” not only survived, but proved to have extraordinary proliferative capacities, not being subject to the Hayflick limit.
From these immortal cells of the tumor that killed Henrietta Lacks 8 months later, the first stable cell line was born, revolutionizing biomedical research. More than 50 million tons of HeLa cells have been distributed worldwide, and used for over 75,000 studies. In 2021, the WHO posthumously awarded Henrietta Lacks a distinction in recognition of her contribution to medical science.

Henrietta Lacks
How did HeLa cells acquire these extraordinary characteristics?
The exceptional capabilities of HeLa cells are the result of a combination of factors. Firstly, HeLa cells have 76 to 80 chromosomes, due to infection with the HPV virus, which has led to the accumulation of errors in the genome as the cells divide. On the other hand, HeLa cells grow even faster than most cancer cells: the researchers attribute this phenomenon to the fact that Henrietta Lacks also suffered from syphilis, a disease that weakens the immune system and allows cancers to develop aggressively. Finally, HeLa cells can divide an infinite number of times thanks to an overexpression of telomerase, which keeps telomeres at a constant length despite the large number of divisions.
Subsequently, researchers developed numerous other cell lines, either from cancer cells, or by “immortalizing” non-cancerous cells. Today, the ATCC (American Type Culture Collection) catalog contains over 4,000 human and animal cell lines, available to researchers worldwide.
Optimizing cell culture conditions
While cell lines help stabilize in vitro culture, culture media are based on fluids such as serum or tissue extracts, leading to great variability and contamination problems.
In the 1950s, Harry Eagle, an American physician and biologist, set out to create a defined, reproducible culture medium that could consistently support cell growth. His research enabled him to identify several essential components for maintaining cells in culture: amino acids, vitamins, mineral salts and glucose. In addition, although Eagle’s aim was to minimize the use of undefined biological components, fetal bovine serum (FBS) was often added to provide growth factors and other nutrients not specifically identified.
Based on these essential components, in 1965 Eagle developed its standardized cell culture medium, Eagle’s Minimum Essential Medium (MEM). MEM served as the basis for other improved culture media, such as DMEM (Dulbecco’s Modified Eagle Medium), which contains higher concentrations of certain nutrients to support the growth of more demanding cells. It is still ubiquitous in laboratories today, due to its ability to support a wide range of cell types.
Alongside the standardization of culture media, culture conditions are also standardized and optimized: development of CO2 incubators, aseptic techniques, use of antibiotics, etc. This standardization of cell culture techniques improves the reproducibility of experiments, and has enabled numerous advances. This standardization of cell culture techniques improves the reproducibility of experiments, and has led to numerous advances.

Bottles of DMEM culture medium
The hybridoma technique
Prior to 1975, antibodies used for research and therapeutic applications were generally produced from the sera of immunized animals.
This method had a number of limitations, including variability, limited availability and low specificity.
In 1975, Georges Köhler and César Milstein developed the hybridoma technique, based on the fusion of myeloma cells, capable of dividing indefinitely, with B lymphocytes from the spleens of immunized mice, capable of producing antibodies.
The fused cells (hybridomas) are then selected in a specific medium that allows only the survival of the fused cells.
This technique enabled the large-scale production of standardized monoclonal antibodies, and earned Köhler and Milstein the Nobel Prize for Physiology or Medicine in 1984.
Conclusion and outlook
Since the discovery of cells at the end of the 17th century, cell culture has constantly evolved to become one of the indispensable technologies in biomedical research and biotechnology. The latest advances in this field continue to transform the scientific landscape: innovative techniques such as microfluidics, 3D culture, organoids and even bioprinting make it possible to model complex tissues and organs, providing increasingly realistic in vitro models for studying the complexity of biological processes and facilitating drug development. Culture media have also seen their share of innovation, to meet the new challenges of biomanufacturing (serum-free media, media without animal products, media for regenerative medicine, etc.).
At the same time, industrial biomanufacturing processes are constantly improving, with increasingly sophisticated bioreactors, continuous culture systems or the use of microcarriers. These innovations promise to improve the large-scale production of therapeutic biomolecules, making them more customizable, lowering production costs and widening access to these biotherapies.
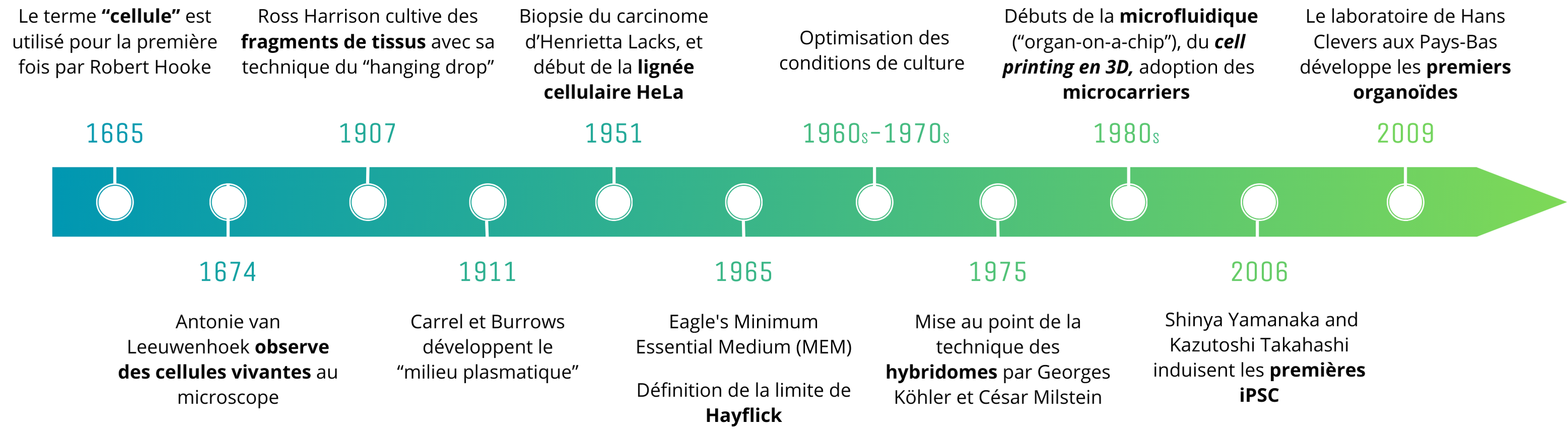
History of major advances in cell culture
MabDesign is an expert in biopharmaceuticals and biomanufacturing. To find out more about these markets, take a look at our various offers. Training courses and conferences to keep you at the cutting edge of knowledge, marketing analyses to understand and segment your markets, business development support to target and expand your customer base, watches to detect opportunities and innovations, and support to finance your innovations: contact us!
Sources
- MabDesign data and expertise
- Millet, Larry Gillette, Martha. (2012). Over a Century of Neuron Culture: From the Hanging Drop to Microfluidic Devices.
- Gu, Z., Fu, J., Lin, H., He, Y. (2020). Development of 3D bioprinting: From printing methods to biomedical applications.
- Hudu, S. A., Alshrari, A. S., Syahida, A., Sekawi, Z. (2016). Cell Culture, Technology: Enhancing the Culture of Diagnosing Human Diseases.
- Jedrzejczak-Silicka, M. (2017). History of Cell Culture.
- Taylor, M. W. (2014a). A History of Cell Culture. In M. W. Taylor (Ed.), Viruses and Man: A History of Interactions (pp. 41-52).
- J.-F. LESESVE (2020) Antonie van Leeuwenhoek, the discoverer of red blood cells
Abbreviations
OoC – Organ-on-chips
WHO – World Health Organization
ATCC – American Type Culture Collection
FBS – Fetal Bovine Serum
MEM – Eagle’s Minimum Essential Medium
DMEM – Modified Eagle’s Medium