MabDesign’s market analyses give you a monthly overview of market dynamics and projects in development for a given indication or technology, to help you better understand the constantly evolving market of biologics and biomanufacturing. This month, an overview of the market and projects currently under development for the prevention of respiratory syncitial virus (RSV).
Respiratory syncytial virus (RSV) is a virus that causes respiratory infections affecting mainly young children, the elderly and immunocompromised individuals. It can cause serious infections of the upper and lower respiratory tracts.
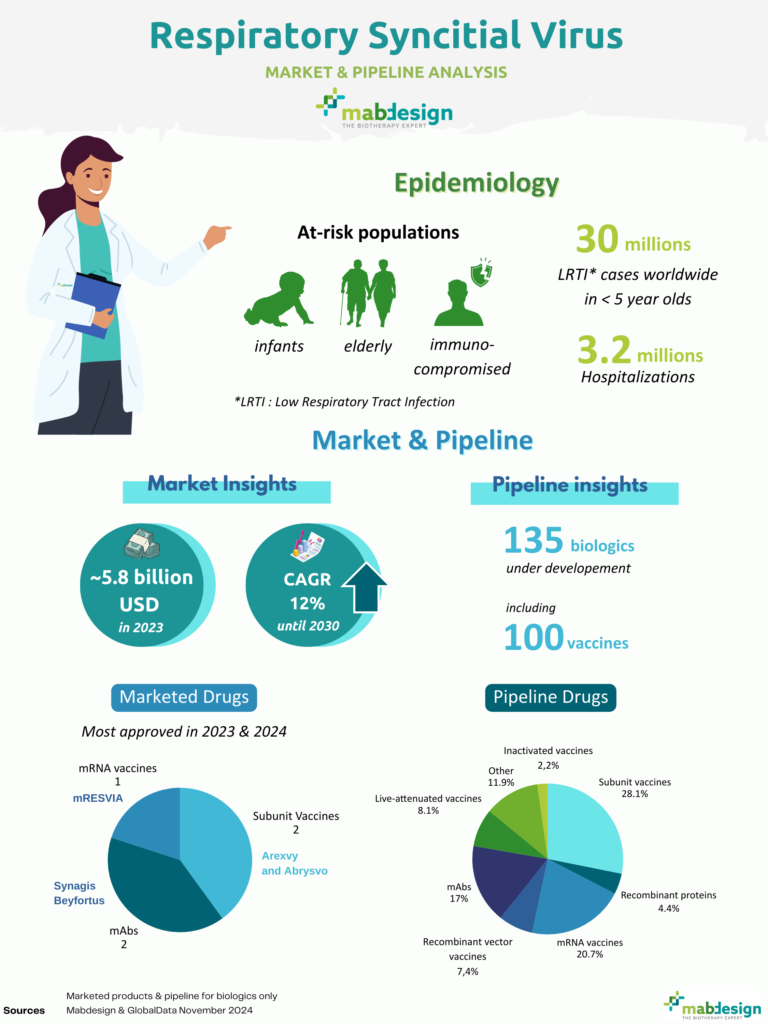
It is transmitted seasonally, with epidemic peaks in autumn and winter: every year, it is responsible for major epidemics of bronchiolitis in newborns, leading to peaks in hospitalisations in paediatric wards. Worldwide, the virus is thought to be responsible for 30 million cases of LRTI and 3.2 million hospitalizations in children under 5 every year.
The virus also represents a significant and probably underestimated burden in the elderly and immunocompromised patients.
Epidemiological data
At-risk populations

30
million LRTI* in children <5 years old
3,2
million hospitalizations
Market and pipeline
The historical product on the market is the monoclonal antibody Synagis, used for prophylaxis in a very limited population of premature newborns. However, between 2023 and 2024, four new products have been approved for the prevention of RSV infections:
- the monoclonal antibody Beyfortus, for passive immunisation of newborns during their first season of RSV circulation
- Arexvy vaccine, indicated for active immunisation of people aged over 60
- the Abrysvo vaccine, indicated for active immunisation of people over 60 and for pregnant women between 32 and 36 weeks of amenorrhoea for passive protection of newborns during the first 6 months of life.
- mRESVIA vaccine for active immunisation of people over 60
Market

Biologics on the market
Pipeline
135
biologics in development, including
100
vaccine products in development
Biologics in the pipeline
The RSV biomedicines pipeline is rich, with 135 unique biomedicines in development, including 100 vaccines.
The emergence on the market of new products for the prevention of RSV infections over the last two years is a major advance in public health. The impact of these new prevention strategies and of the possible emergence of new products from the pipeline will remain to be measured in the years to come.
Want to find out more?
MabDesign has acquired and cultivates its expertise in all market segments associated with biopharmaceuticals and biomanufacturing. Our team of consultants is at your disposal to support you in all your biotherapy-related projects. Feel free to explore our range of services, including our marketing and strategic analyses, if you’d like to find out more!
Sources: MabDesign & GlobalData
Date – November 2024