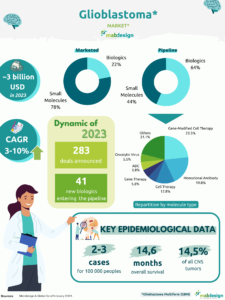
Each month, MabDesign’s market analyses give you an overview of market dynamics and projects in development for a given indication or technology, to help you better understand the constantly evolving market for biomedicines and biomanufacturing. This month, an overview of antibody-oligonucleotide conjugate (AOC) projects currently in development.
The emergence of antibody-drug conjugates, ADCs, marked a major advance in combining chemotherapy with monoclonal antibodies to create a powerful immunotherapeutic tool. Their precision and efficacy make them an essential pillar in the development of cancer treatments.
In parallel, nucleic acid-based therapies have attracted attention for their ability to modulate cellular functions at the genetic level using nucleotide sequences. These therapies can produce highly targeted and long-lasting therapeutic effects for hereditary and acquired diseases, such as genetic disorders, cancers, viral infections and neurological conditions.
Building on the success of ADCs, antibody-oligonucleotide conjugates (AOCs) have emerged as a new fusion, combining the precision of nucleic acids with the targeted delivery capability of antibodies.
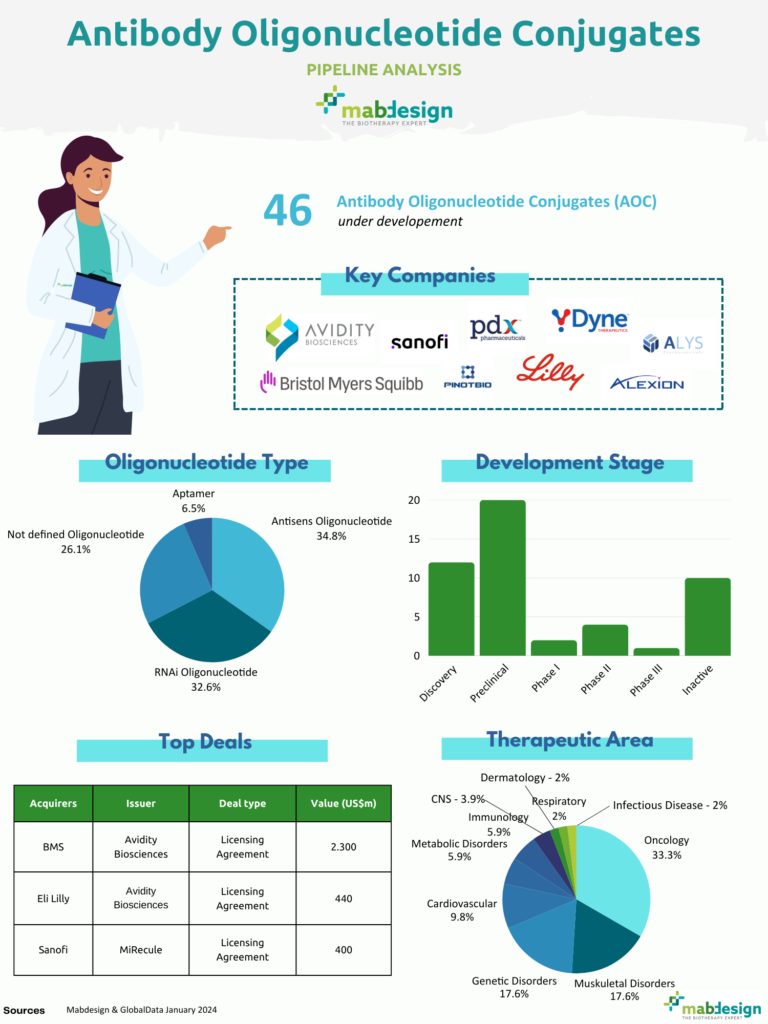
Several pharmaceutical companies are conducting clinical trials on AOC drugs, including Avidity, which is developing the first AOC to enter the clinic, reaching phase III in May 2024. Despite these advances, research into AOCs is still in its infancy, with 46 AOCs in development in the pipeline. Of these, 34.8% are antisense oligonucleotides (ASOs) and 32.6% are RNA interference (RNAi).
Oligonucleotide types
Number of AOCs in the pipeline
Key players

The antibody-oligonucleotide conjugate (AOC) industry is experiencing rapid momentum, as evidenced by recent partnerships between biotechs such as Avidity Biosciences and major pharmaceutical companies such as Bristol Myers Squibb and Eli Lilly.
- In 2019, Eli Lilly entered into a licensing and collaboration agreement with Avidity Biosciences, investing $35 million to develop new drugs based on antibody-oligonucleotide conjugates in immunology and other indications.
- Avidity announced a collaboration with Bristol Myers Squibb in 2023, with an initial investment of $100 million to develop five cardiovascular targets using Avidity’s AOC technology.
- In October 2022, Sanofi and MiRecule announced a strategic collaboration and exclusive licensing agreement to develop and commercialise an antibody-RNA conjugate (ARC) for the treatment of facioscapulohumeral muscular dystrophy (FSHD).
These collaborations illustrate the growing interest of major pharmaceutical companies in AOCs.
Acquirers | Issuer | Deal type | Value (US$m) |
|---|---|---|---|
BMS | Avidity Biosciences | Licensing agreement | 2.300 |
Eli Lilly | Avidity Biosciences | Licensing agreement | 440 |
Sanofi | MiRecule | Licensing agreement | 400 |
The therapeutic areas targeted by the antibody-oligonucleotide conjugates in the pipeline are relatively varied, with a predominance in oncology, but also for pathologies affecting muscles and genetic diseases.
In terms of the progress of these projects, more than half are in the non-clinical phases of development. Only 6 AOCs are currently in clinical phase.
Development phases and therapeutic areas
Want to know more ?
MabDesign has acquired and cultivated expertise in all market segments associated with biomedicines and biomanufacturing. Our team of consultants is available to support you in all your projects related to the biotherapeutics sector. Please feel free to explore our range of services, including our marketing and strategic analyses, if you’d like to find out more!
Sources : MabDesign & GlobalData
Date – January 2025