Biopharmaceuticals, or biotherapies, are becoming increasingly important on the market, and offer immense hope for the treatment of many diseases. From the first discoveries of vaccines to the recent development of cell and gene therapies and regenerative medicine, looking back at biologics’ history lets us have a better understanding of the revolution that they provoked in the pharmaceutical industry and in modern medicine .
Fundamental discoveries and definition of biopharmaceuticals
It all began at the end of the 19th century, marked by fundamental discoveries in biology and chemistry. Historically, the treatment of illnesses was based on the use of natural remedies: this tradition was overturned in 1869 with the arrival of the first synthetic drug, chloral hydrate. This breakthrough was closely followed by the development of numerous other “small chemical molecules” derived from natural remedies, such as aspirin in 1897.
At the same time, the history of biomedicines began with vaccines: following the historic experiments of Edward Jenner and Louis Pasteur, vaccines and serum-based therapies were developed. However, these initial experiments faced major challenges, particularly in terms of understanding of the underlying biological mechanisms of action, safety, and social acceptance.
It was at this time that the first definition of “biologic”appeared: they are defined as drugs of “natural” origin (plants or animals), as opposed to those obtained by chemical synthesis.
Larger and more complex than their chemical counterparts, the first biomedicines faced a major obstacle: production difficulties, particularly in terms of yield and safety. Examples include the growth hormone hGH, which had to be extracted from cadavers, or insulin, which required extraction from pig or bovine pancreas.
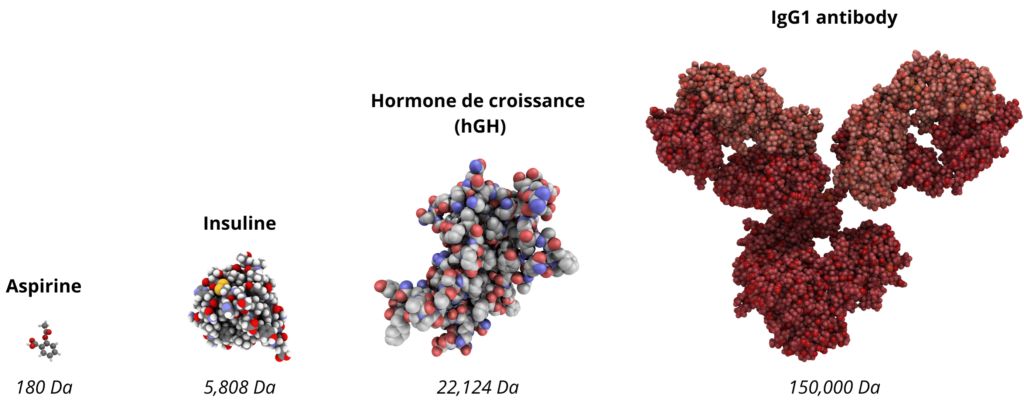
Structures and sizes of aspirin, insulin, hGH and IgG1 molecules. Biopharmaceuticals are larger and more structurally complex than small molecules.
The biotechnologies revolution
The 20th century was marked by advances in genetic engineering and molecular biology: the foundations of biomedical therapy were laid with the decoding of DNA structure (1953), and the development of genetic recombination and molecular biology techniques (1970s). These techniques then became essential tools for the design and production of biomedicines.
These advances, combined with the growing body of knowledge about the immune system and biological processes, have opened up new prospects for the development of innovative therapies, notably in improving production methods and yields. They also led to a redefining of biologics Biopharmaceuticals are from then defined as agents whose components or origins are natural, and which are constructed or produced with the help of bioengineering or biotechnology.
The 70s and 80s saw the development of new biotherapies: one of the most striking is recombinant human insulin produced by genetic engineering. This was a major advance in the treatment of diabetes: previously, 2 tonnes of pig pancreas were needed to produce 200g of pork insulin. Because recombinant insulin can be produced in large quantities, it is accessible to many more patients. It is also more effective, with fewer side effects.
Nevertheless, the production of these “large molecules”, 200 to 1000 times larger and more complex than small chemical molecules, remains a major challenge even today.
Monoclonal antibodies: the advent of biomedicines
The development of hybridoma production technique, developed by Georges Köhler and Cesar Milstein in 1975 and awarded a Nobel Prize, marks the arrival of the monoclonal antibodies (mAbs): this technique enables the production of a set of identical antibodies (a clone) directed against a specific antigen. Compared with polyclonal antibodies (several different clones) derived from convalescent patients or animal sera, antibodies produced in this way are homogeneous and mono-specific.
In 1985, the first monoclonal antibody was marketed for clinical use: muromonab-CD3, for the treatment of transplant rejection. In the years that followed, the arrival of mAbs marked a turning point in the treatment of autoimmune diseases and cancers, providing therapeutic solutions for rheumatoid arthritis, certain lymphomas and breast cancers, and Crohn’s disease. For autoimmune diseases, mAbs can target specific proteins involved in the excessive immune response, thereby reducing inflammation and tissue damage without necessarily causing strong systemic immunosuppression. In oncology, they provide more targeted treatments, based on the markers expressed by a given tumor. They are therefore more effective and better tolerated than conventional treatments such as chemotherapy, and pave the way for personalized medicine in cancer treatment.
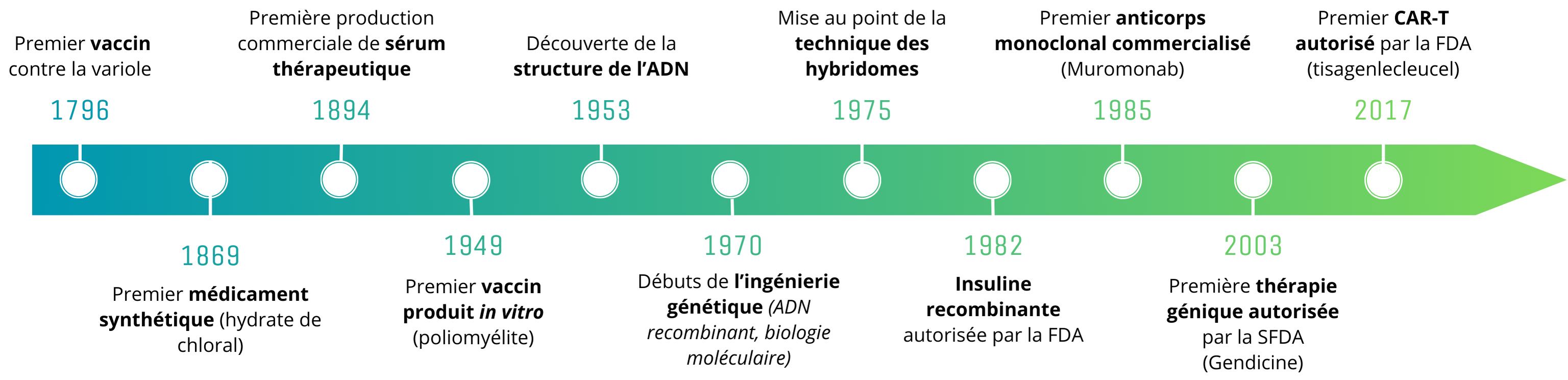
Chronology of major advances in biomedicine
Expanding the range and scope of biomedicines
Today, biomedicines are used to treat diseases as diverse as diabetes, cardiovascular disease, neurological disorders and viral infections. This expansion of the field of application has been made possible by numerous innovations leading to new types of biomedicines.
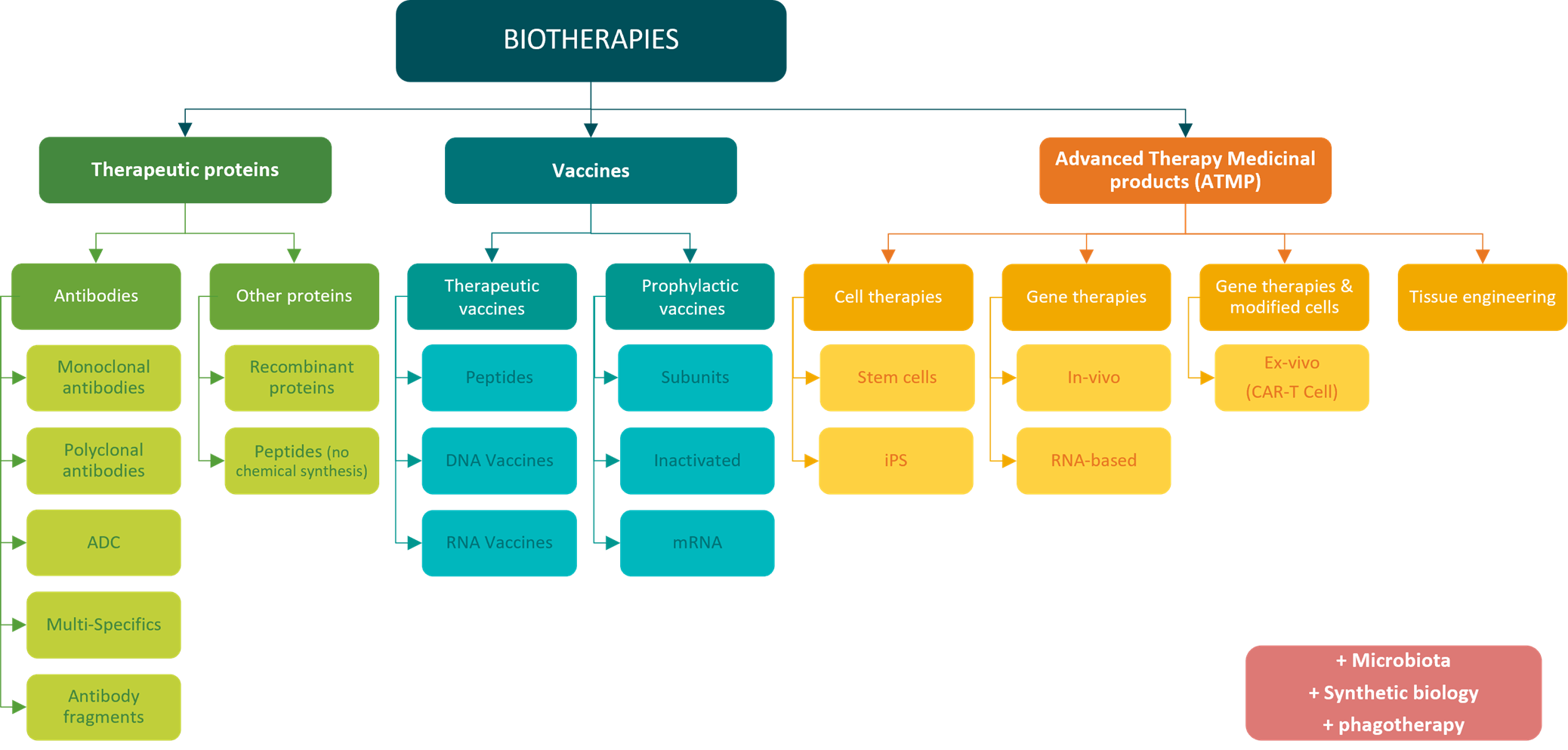
Classification of biotherapies into major families – MabDesign
Therapeutic proteins are diversifying, with the emergence of many new technologies: multispecific antibodies, antibody-drug conjugates (ADCs), antibody fragments, peptides, recombinant proteins and more.
The vaccines are also a source of innovation A wide range of technologies has been developed over the years, in addition to conventional attenuation and inactivation technologies, such as subunit, mRNA and DNA vaccines. The diversity of our prophylactic vaccine arsenal was recently in the spotlight during the COVID-19 pandemic (see our immunowatch special edition COVID-19). On the other hand, beyond historically prophylactic approaches, vaccines are now also emerging as a potential therapeutic tool, mainly in oncology.
New families of biomedicines are emerging, with Advanced Therapy Medicinal Products (ATMPs), combiningtissue engineering and gene and cell therapies. These represent a promising new treatment: correcting the genetic defects at the root of certain diseases, replacing damaged cells with healthy ones, or enhancing the therapeutic power of our cells. CAR-T cells, for example, are engineered by genetically modifying a patient’s own T lymphocytes to express chimeric receptors (CARs) specifically designed to target and destroy cancer cells. Once reinjected into the patient’s body, CAR-T cells can precisely target cancer cells and trigger a highly effective immune response against the tumor.
These approaches offer new treatment possibilities for a wide range of diseases, including genetic disorders, blood diseases, neurological disorders and cancers. Although these therapies are still under development, and still face many challenges, they represent a major field of investigation today. With a considerable and dynamic pipeline of ATMP candidates in development, we are regularly witnessing new therapeutic breakthroughs.
February 2024
First gene therapy approved by the EU
In February 2024, the European Commission granted a marketing authorization for a gene therapy based on CRISPR/Cas9 technology (CASGEVY®), for the treatment of sickle cell disease and transfusion-dependent beta-thalassemia. This is the first gene therapy approved by the European Union, and it could be administered to over 8,000 patients.
Innovations such as phagotherapy, microbiotic therapies and synthetic biology are also appearing in the growing landscape of biomedicines.
Challenges and prospects
Today, compared to small chemical molecules, relatively few biomedicines are on the market. Nevertheless, their contribution to the fight against many diseases is tremendous. This distribution will likely change in the future, since more than half the molecules currently in development are biotherapies.
However, challenges remain, particularly in terms of accessibility, costs and regulations. Healthcare systems have to cope with the complexities of managing these treatments, which are much more expensive than more conventional therapies. The bioproduction of these molecules also remains a major challenge for the sector, and an important vector for innovation.
The biopharmaceuticals sector is constantly driven by innovation, making definitions and categorizations fluid, to say the least. New approaches are continually being developed, making the field both exciting and demanding in terms of knowledge of the market and emerging technologies.
To find out more about this market, take a look at MabDesign’s various offers. Training courses and conferences to keep you at the cutting edge of knowledge, marketing analyses to understand and segment your markets, business development support to target and expand your customer base, watches to detect opportunities and innovations, and support to finance your innovations: contact us!
Sources
- MabDesign data and expertise
- The History of Biologics: Problems, Perseverance, and Potential-Beckman
- A fascinating story of the discovery & development of biologicals for use in clinical medicine – Malaviya et al., 2018
- The Biologics Revolution in the Production of Drugs – Fraser Institute
- Biopharmaceutical Manufacturing: Historical Perspectives and Future Directions, Alana C. Szkodny and Kelvin H. Lee, Annual Review of Chemical and Biomolecular Engineering 2022 13:1, 141-165
- The bioproduction of monoclonal antibodies, Joubert et al., 2019
- Jones AW. Early drug discovery and the rise of pharmaceutical chemistry. Drug Test Anal. 2011 Jun;3(6):337-44. doi: 10.1002/dta.301. PMID: 21698778.
- Biotechinfo.fr
Abbreviations
hGH: human growth hormone
mAb: monoclonal antibody
FDA: Food and Drug Administration
SFDA: Chinese State Food and Drug Authority
CAR: chimeric antigen receptor
ADC: antibody-drug conjugates
ATMPs: advanced therapy medicinal products