Every month, MabDesign’s market analyses provide you with an overview of market dynamics and projects in development for a specific indication or technology, to help you better understand the constantly evolving market for biomedicines and biomanufacturing.
The first edition of our Innovations for Cell and Gene Therapies (ICGT) congress, organized in collaboration with the French Society of Cell and Gene Therapy (SFTCG) will take place on May 22, 2024, in Paris. In the meantime, this month we share an overview of the cell and gene therapy market.
Advanced therapy medicinal products (ATMPs) explore new therapeutic approaches based on genome modification or the manipulation of patient or healthy donor cells. They represent a major transition from traditional medicine to a more personalized, targeted approach. These innovative products are accelerating the development of biotherapies, and currently account for around a third of all biopharmaceuticals under development .
The market for in vivo gene therapy
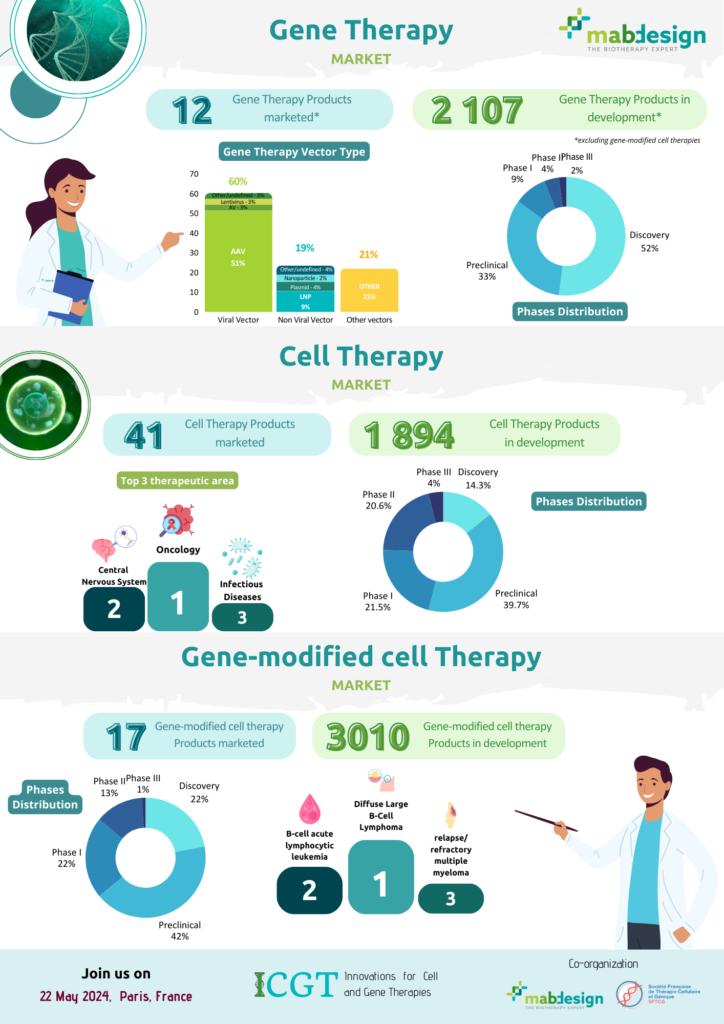
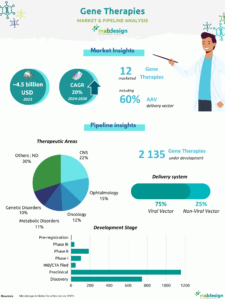
In vivo gene therapy products (excluding genetically modified cell therapies such as CAR-T) are few on the market, with just 12 products currently available. On the other hand, the pipeline is quite extensive, with 2107 gene therapies in development. The vast majority of projects are in the early stages of development, with only 15% in clinical phases. In terms of vectors used, viral vectors, and in particular AAVs(adeno-associated viruses), are the technology most frequently chosen for projects in the pipeline.
*excluding genetically modified cell therapies
In vivo gene therapies in development, by phase
Vector types (pipeline)
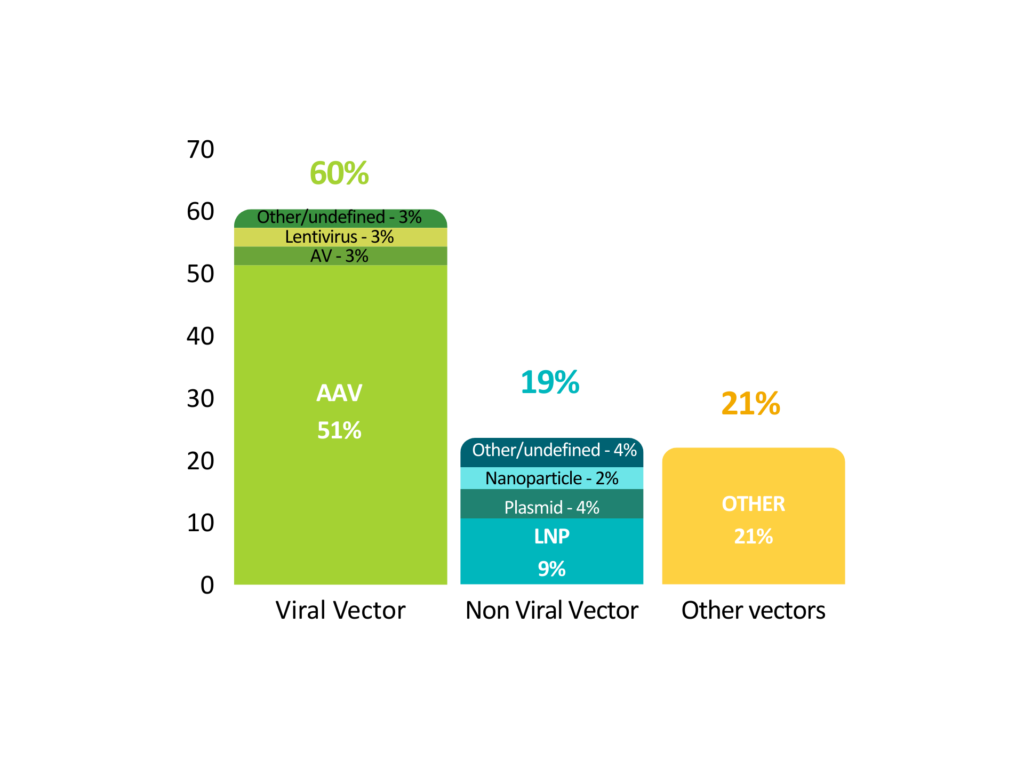
AV: adenovirus; AAV: adeno-associated virus; LNP: lipid nanoparticles
The market for gene-modified cell therapies
Gene-modified cell therapies (or ex-vivo gene therapies), such as CAR-T cells, are still few on the market: there are currently 17 products available. By contrast, the pipeline is very rich, with over 3,000 genetically modified cell therapies in development. Projects are in relatively advanced stages of development, with 36% in clinical phases. In terms of targeted indications, the first three are blood cancers : diffuse large B-cell lymphoma, acute B-cell lymphocytic leukemia, and relapsed/refractory multiple myeloma.
Gene-modified cell therapies in development, by phase
Top 3 indications (pipeline)

The Cell Therapy Market
More cell therapy drugs are on the market: there are currently 41 marketed products and 1,894 cell therapies in development. Compared with gene therapies, 46.1% of cell therapies in development are in clinical phases. The most targeted therapeutic areas are oncology, the central nervous system and infectious diseases.
Cell therapies in development, by phase
Top 3 therapeutic areas (pipeline)

Want to find out more?
In 2024, in collaboration with the SFTCG, MabDesign is launching the first edition of the Innovations for Cell and Gene Therapies (ICGT) congress! You canregisternow, or contact us for more information.
MabDesign has acquired and cultivates its expertise in all market segments associated with biopharmaceuticals and biomanufacturing. Our team of consultants is at your disposal to support you in all your biotherapy-related projects. Feel free to explore our range of services, including our marketing and strategic analyses, if you’d like to find out more!
Sources: MabDesign, GlobalData
Date – March 2024