Advanced therapy medicinal products (ATMPs) are emerging in a scientific and medical context characterized by significant advances in our understanding of the molecular and cellular mechanisms of disease. This revolution follows on from discoveries in genetics, molecular biology and immunology, opening up new perspectives for the treatment of diseases. ATMPs thus explore new therapeutic approaches based on genome modification, manipulation of patient or healthy donor cells and other innovative methods, representing a major transition from traditional medicine to more personalized and targeted medicine.
Classification and mechanisms
An Advanced Therapy Medicinal Product (ATMP) is a class of drug characterized by the use of innovative technologies to treat serious diseases. These therapies exploit approaches such as gene therapy, cell therapy and other methods of genetic manipulation to specifically target the molecular causes of disease.
ATMPs differ from conventional therapeutic drugs in that they act on the molecular cause of a disease, rather than treating the symptoms. They offer potential solutions for diseases that were previously considered incurable.
The first applications of gene therapy were explored as early as the 1980s, but the field took off significantly in the 2000s with improved genetic manipulation techniques and advances in our understanding of stem cells.
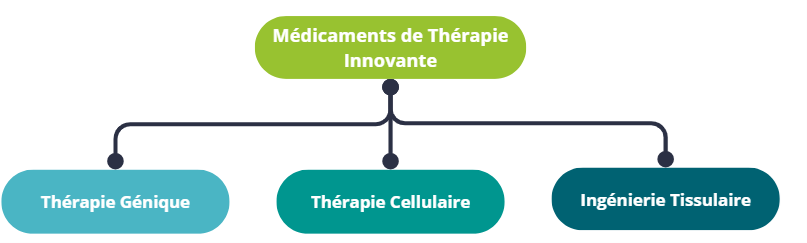
There are several broad categories of ATMPs, the main ones being gene therapy and cell therapy. ATMPs also include tissue engineering and technologies combining products derived from the manipulation of genes, cells or tissues, and medical devices.
ATMPs classification
Gene therapy
Gene therapy aims to introduce, repair or replace genes in the patient’s cells. It can be used to treat diseases by modifying the genetic material of cells, offering a targeted approach to correcting the genetic abnormalities at the root of various disorders.
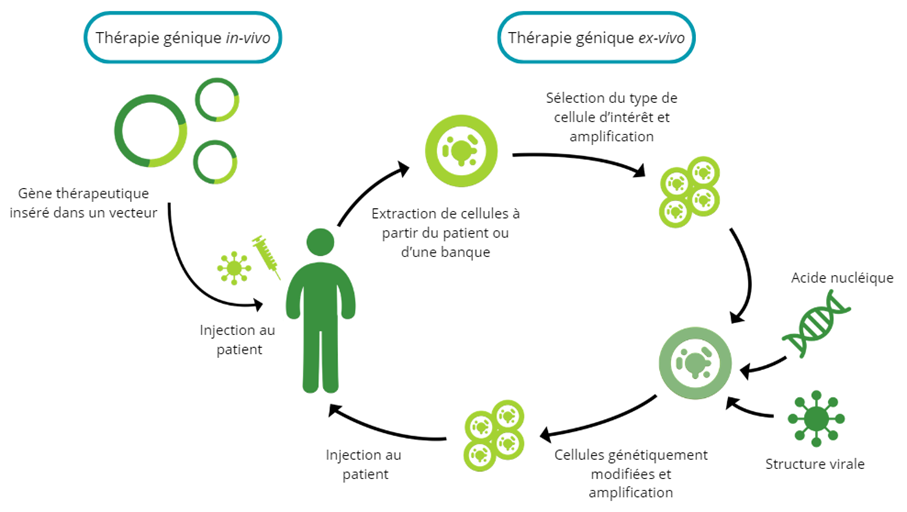
There are two methods of administering genetic material as a drug: ex-vivo or in-vivo. The in-vivo strategy involves direct administration to the patient, without manipulation of cells outside the body. With ex-vivo gene therapy, targeted cells are first harvested from the patient (autologous) or from a healthy donor (allogeneic) before being genetically modified and reinjected . These ex-vivo gene therapies are generally referred to as gene-modified cell therapies.
In both cases, gene material is delivered to the cell using a vector. There are several types of vector, of which modified viruses are the most commonly used. These viral vectors are generally designed to eliminate their ability to cause disease, while retaining their capacity to penetrate cells. Some common types of viral vector include retroviruses, lentiviruses, adenoviruses and adeno-associated viruses. There are also non-viral vectors, which can include lipids or polymers, for example. They have the advantage of minimizing the risks associated with the use of viruses and of being able to carry more genetic material, but they can be less effective in cell penetration.
In terms of clinical applications, gene therapies have provided solutions in a variety of therapeutic areas, including the treatment of cancers, muscular diseases and ophthalmic disorders. The first gene therapy drug was approved in China in 2003: under the trade name Gendicine, this adenovirus is indicated for head and neck cancer. More recently, Zolgensma was the first gene therapy treatment for a neuromuscular disease to receive marketing authorization in Europe, Japan and the USA. It can be used to treat cases of infantile spinal muscular atrophy.
Mechanisms of in-vivo and ex-vivo gene therapy
Cell therapies
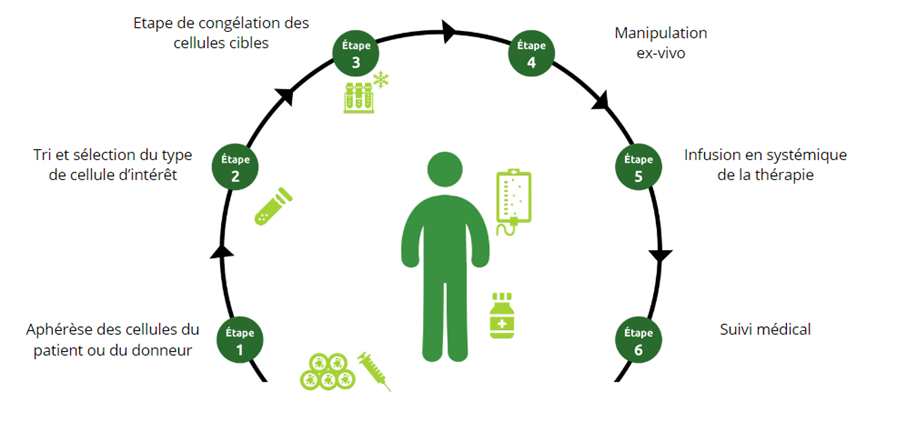
Cell therapy involves the manipulation of cells, often stem cells, to regenerate or replace damaged cells. The treatment differs from a traditional transplant in which the cells are not significantly manipulated, and in which the cells have the same essential function as in the donor. Cell therapy drugs are cells or tissue that have been manipulated or reprogrammed prior to administration to the patient.
Pluripotent stem cells can produce any cell type in the human body. They are also capable of multiplying and being maintained outside the human body for long periods, making them an ideal tool for the development of cell therapies.
Cell therapy can also be carried out with multipotent stem cells, which can differentiate into a smaller number of cell types than their pluripotent counterparts. Mesenchymal stem cells are the most widely used, and are found throughout the body in adipose tissue, bone marrow, organ support tissues, as well as in bone, cartilage, muscle, and so on.
The use of skin stem cells to treat burn victims has been practiced since the 1970s. Approved cell therapies are positioned in the treatment ofhematological malignancies and in ophthalmology. Clinical trials are varied, especially for treatments with mesenchymal stem cells, and concern rheumatology (osteoarthritis, rheumatoid arthritis), muscle degeneration (myopathies), cardiology, diabetes, autoimmune diseases, etc.
Cell therapies also include non-stem cells, including immune system cells such as dendritic cells and cytotoxic T lymphocytes, as well as other cell types such as keratinocytes, fibroblasts and myoblasts.
Mechanisms of cell therapy
Tissue-engineered products
A tissue-engineered product contains manipulated cells or tissues with properties that enable them to regenerate, repair or replace human tissue. Tissue engineering stems from bioengineering, which aims to develop methods for creating functional biological tissues in the laboratory.
The main steps in tissue engineering generally involve selecting or creating biomaterials compatible with the body, culturing specific cells in the laboratory,assembling these cells into three-dimensional structures (called scaffolds or matrices), and finally transplanting these structures into the patient’s body. These structures are designed to mimic the structure and function of natural tissues, promoting regeneration and integration into the surrounding tissue. Applications mainly concern the repair of chronic, acute, degenerative or traumatic tissue damage.
ATMPs future successes and challenges
Targeted and personalized therapies
Advanced therapy medicinal products represent a significant advance in the medical field, thanks to their ability to directly target the biological mechanisms responsible for pathologies. This capability stems from the personalized nature of ITMs, which can be designed to adapt to each patient’s unique genetic and biological characteristics. By precisely understanding the molecular mechanisms responsible for a given disease in a given patient, ATMPs can be modulated to correct genetic abnormalities, stimulate cell regeneration or regulate or enhance specific biological processes.
This targeted approach offers a number of advantages, including greater treatment efficacy and a reduction in the undesirable side effects associated with less precise drug interventions (chemotherapy, for example).
By acting directly on the fundamental cause of disease, ATMPs pave the way for more powerful, tailored treatments, promising a revolution in the management of various diseases.
Challenges and prospects
While ATMPs open up new therapeutic prospects, their use involves challenges in terms of safety, biomanufacturing (costs, standardization, etc.), administration, patient access and regulation..
The genetic and cellular manipulation associated with ATMPs entails risks that are crucial to understand and control.
ATMPs have the capacity and function to remain in the human body, and include living material that develops and interacts with the other components of the organism. Unexpected immune responses, unanticipated genetic mutations and other adverse reactions require close monitoring to assess long-term safety of ATMPs. As this class of drugs is a recent development, we currently lack the benefit of hindsight, especially given their variety and the complexity of the technical and biological processes on which they are based.
The technical complexity of ATMPs also stems from their personalized nature. Standardization of technical procedures represents a major challenge, requiring advanced expertise to ensure the reproducibility and quality of ATMPs on a large scale, without compromising the personalization that is their strength. On the other hand, some types of ATMPs require complex handling and administration procedures, which can pose logistical problems and a need for training of medical staff.
Finally, the high cost of developing ATMPs is one of the key challenges facing the sector. Researching and developing these therapies requires considerable investment in human resources and specialized infrastructures. Production costs for viral vectors, modified cells and other ATMPs-specific components are also substantial. These high costs are reflected in the final cost of treatment, raising concerns about affordability for a wide range of patients.
ATMPs market overview
MabDesign is constantly developing its expertise in different classes of biomedicines, including the ATMPs market and more specifically the gene therapy and cell therapy segments.
These innovative products are accelerating the development of biotherapies, and currently account for around a third of all biopharmaceuticals in development .In all, some 7,000 ATMPs are currently in the development pipeline, combining gene therapies (including genetically modified, or ex-vivo, cell therapies ) and cell therapies .
On the market :
in development :
ATMP Pipeline
Gene therapy products are widely investigated, with 5,075 products in development and 28 having received marketing authorization. Ex-vivo gene therapies account for almost 60% of gene therapies in development. CAR-T cells are the ex-vivo gene therapies with the most marketing approvals. The first CAR-T cell treatment was marketed by Novartis under the trade name Kymriah, approved by the FDA in 2017. Since then, various CAR-T cell therapies have been approved and positioned in hematology, notably for the treatment of lymphoma and myeloma.
More and more cell therapy drugs are on the market: there are currently 41 commercialized products. Very recently, new cell therapies, called TILS for ” Tumor Inflating Lymphocytes “, have been developed. A first product, Iovance’s Lifileucel, has been approved by the FDA for patients with metastatic melanoma. There are currently 1,894 cell therapies in development, 731 of which use stem cells. The therapeutic areas targeted by stem cell-based products are mainly neurodegenerative diseases, followed by cardiovascular diseases and muscular degeneration.
The arrival of Advanced Therapy Medicinal Products offers solutions deemed revolutionary and promising in patient care, notably through personalized treatments targeting the underlying causes of disease. Significant steps have already been taken to encourage the development of these ATMPs, and these must be maintained: investing massively in research and development via public or private funding, developing regulations to guarantee safety while speeding up time-to-market, and reducing production costs to enable access to a large number of patients.
If you’re looking for support in your gene therapy or cell therapy projects, don’t hesitate to exploreour range of servicesand contact us for a chat. We’d be delighted to use our expertise to help you achieve your goals!
In 2024, Mabdesign is teaming up with the Société Française de Thérapie Cellulaire et Génique (SFTCG) to organize the first congress on Innovations in Gene and Cell Therapy. This one-day event enables academic and industrial experts in the field to discuss recent advances.
Sources
- MabDesign expertise
- GlobalData 2024
- Société Française de Thérapie Cellulaire et Génique
- Thérapie cellulaire et thérapie génique, Inserm
- Les Médicaments de Thérapie Innovante, BioMérieux
- Les produits biologiques, ANSM
- La France et les médicaments de thérapie innovante, LEEM et MabDesign