From Paul Ehrlich’s early “magic bullet” concept to today’s approved therapeutics, Antibody-Drug Conjugates (ADCs) represent a major breakthrough in targeted cancer therapy. By combining the selectivity of monoclonal antibodies with the potency of cytotoxic agents, ADCs aim to eliminate tumor cells while sparing healthy tissues. This article dives into the evolution, design strategies, and key challenges of these complex biologics.
Historical background of ADCs
The history of Antibody-Drug Conjugates (ADCs), revolutionary biomedicines in oncology, goes back to the visionary work of Paul Ehrlich, a German scientist at the beginning of the 20th century. Paul Ehrlich is often regarded as the father of modern immunology and chemotherapy. His concept of the ‘magic bullet’ laid the foundations for targeted therapy, an idea that resonates particularly well with ADCs.
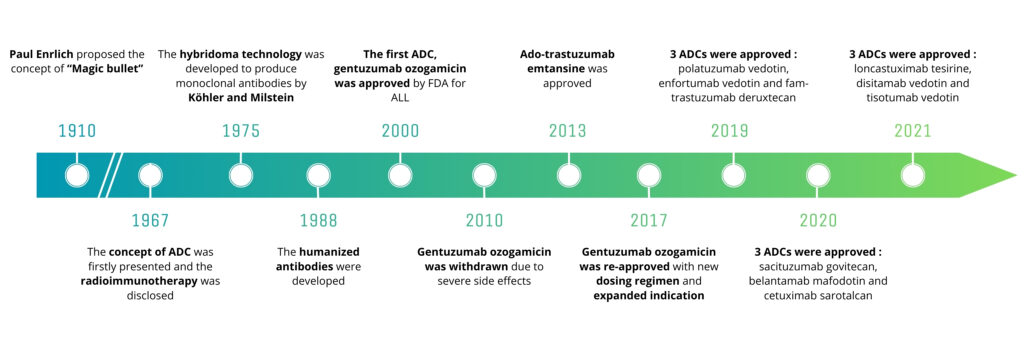
Figure 1: Major scientific breakthroughs that paved the way for the development of ADCs, adapted from Zhiwen Fu, Shijun Li, Sifei Han, Chen Shi & Yu Zhang, Signal Transduction and Targeted Therapy, 2022.

Paul Ehrlich – (1854-1915)
At the beginning of the 1900s, Ehrlich was working on ways to treat infections and diseases without damaging healthy tissues. He proposed the idea of a “magic bullet” — a compound capable of specifically targeting pathogens or diseased cells (such as cancer cells) while sparing healthy tissues. At the time, there was no advanced technology to bring this idea to reality, but his vision remained a major source of inspiration in the development of modern medicine. Ehrlich also made crucial discoveries in the field of antibodies—proteins capable of specifically recognizing antigens carried by pathogens such as bacteria and viruses. He suggested that antibodies could be used to deliver therapeutic agents directly to specific targets within the body, a concept that foreshadowed the mechanism of action of ADCs. It was only several decades after Ehrlich’s visionary proposals that advances in immunology and biotechnology enabled the conjugation of antibodies to cytotoxic agents.
In the 1980s, the first clinical trials involving ADCs were initiated (for example, targeting CD33 in acute myeloid leukaemia), but it was not until the 2000s that the first ADCs were approved by regulatory agencies—such as gemtuzumab ozogamicin, indicated for the treatment of acute myeloid leukaemia. Since the beginning of the 21st century, ADCs have become essential tools in the fight against cancer. Researchers continue to refine ADC design to enhance specificity (and therefore reduce toxicity) and improve therapeutic efficacy. Paul Ehrlich’s idea has thus become a reality—more than a century after his initial intuitions.
The first ADC trials began in the 1980s, but it was not until the 2000s that the first ADCs were approved by drug regulatory agencies, such as gemtuzumab ozogamicin indicated for acute myeloid leukaemia. Since the beginning of the 21st century, ADCs have become essential tools in the fight against cancer. Researchers continue to refine the design of ADCs to make them more specific and more effective.
Paul Ehrlich’s idea has thus become a reality, more than a century after his first intuitions.
Structure and mechanism of action of ADCs
An Antibody-Drug Conjugate (ADC) is a targeted therapy that harnesses the specificity of a monoclonal antibody for an antigen preferentially expressed on tumour cells. This biopharmaceutical enables the selective delivery of a highly potent cytotoxic agent directly to cancer cells.
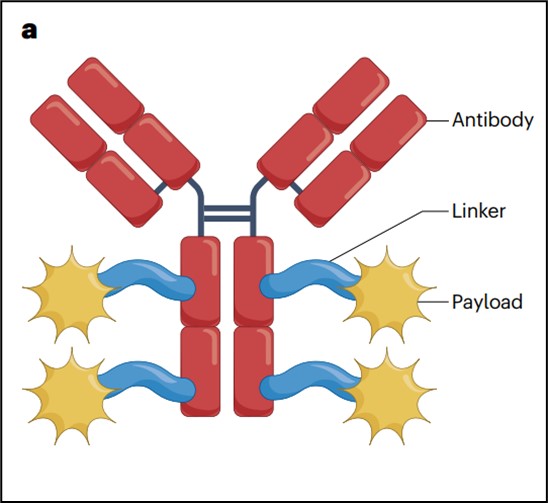
Figure 2: Structure of an ADC, adapted from Dumontet C, Reichert J, Senter P, Lambert J, Beck A. Nature Reviews Drug Discovery, 2023.
An ADC is a “prodrug”, meaning it is administered in an inactive form and subsequently metabolized to produce an active metabolite—that is, the pharmacologically active form of the ADC. This property helps reduce systemic toxicity to healthy cells, in contrast to traditional chemotherapies.
First, the antigen targeted by the ADC must be well characterized, with high and homogeneous expression on tumour cells and low expression on healthy tissues. Interestingly, complete homogeneity of antigen expression is not absolutely required if compensatory mechanisms such as the bystander effect (see box below) are present. However, antigen homogeneity remains an important criterion in the selection and optimization of ADC candidates.
Multiple ADCs can be approved for the same cellular target, such as the HER2 antigen (with four approved ADCs), underscoring the challenge of identifying novel therapeutic targets for innovative ADC development.
An ADC is a “prodrug”, meaning it is administered in an inactive form and subsequently metabolized to produce an active metabolite—that is, the pharmacologically active form of the ADC. This property helps reduce systemic toxicity to healthy cells, in contrast to traditional chemotherapies.
First, the antigen targeted by the ADC must be well characterized, with high and homogeneous expression on tumour cells and low expression on healthy tissues. Interestingly, complete homogeneity of antigen expression is not absolutely required if compensatory mechanisms such as the bystander effect (see box below) are present. However, antigen homogeneity remains an important criterion in the selection and optimization of ADC candidates.
Multiple ADCs can be approved for the same cellular target, such as the HER2 antigen (with four approved ADCs), underscoring the challenge of identifying novel therapeutic targets for innovative ADC development.
The structure of an ADC consists of three main components:
- Monoclonal antibody: which specifically recognizes and binds to a target antigen expressed on the surface of cancer cells.
- Cytotoxic payload: a highly potent chemical agent designed to induce apoptosis in cancer cells. These molecules are too toxic to be administered systemically in their free form.
- Linker: which connects the antibody to the cytotoxic payload. The linker is engineered to remain stable in systemic circulation but to degrade once the ADC is internalized into the target cell, thereby releasing the cytotoxic drug intracellularly.
The antibody–linker junction plays a critical role: on one hand, it determines the method of bioconjugation, depending on the amino acid used for payload attachment, and defines the Drug-to-Antibody Ratio (DAR)—i.e., the number of payload molecules per antibody. On the other hand, antibody conjugation influences ADC stability and impacts off-target toxicity.
The linker–payload bond is also a key determinant of the payload type, defines the remaining chemical group on the payload to ensure its functionality, contributes to off-target toxicity, and affects the molecule’s hydrophilicity.
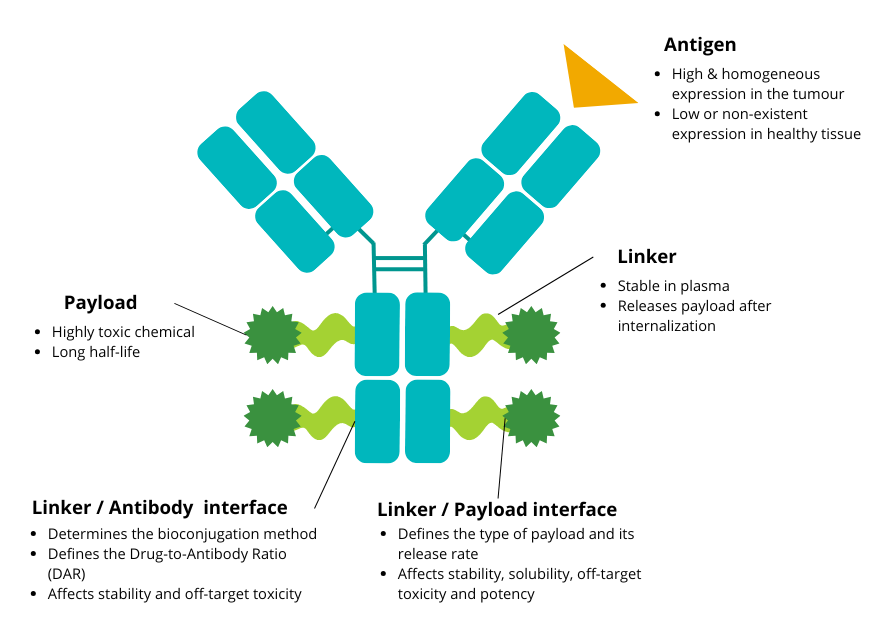
Design of an antibody-drug conjugate and recommended biological properties.
Characteristic of antigen, antibody, linker-antibody attachment, linker, linker-payload attchment and payload are detailed. The antibody Fc part is implied in half-life and immunogenicity. The Fab part controls affinity and avidity to the targeter antigen.

Figure 3: Mechanism of action of ADCs upon binding to an antigen-expressing cell – Adapted from Dumontet C, Reichert J, Senter P, Lambert J, Beck A. Nature Rev Drug Discovery 2023.
The main steps involved in the internalization and degradation of ADCs are illustrated in Figure 3 and include:
(1) Specific binding of the ADC to the target antigen
(2) Internalization of the ADC–antigen complex
(3) Trafficking to the endosome and then to the lysosome: degradation of the antibody and cleavage of the linker to release the payload
(4) Release of the payload into the cytoplasm
(5) Interaction of the payload with its cellular targets—such as DNA, microtubules, or topoisomerase I—depending on its mechanism of action
(6) Release of the payload into the tumour microenvironment
(7) Diffusion to neighbouring cells (bystander killing effect)
Focus: the bystander effect of ADCs
The bystander effect of ADCs occurs when the cytotoxic payload, once released inside a targeted cancer cell, is expelled into the tumour microenvironment and subsequently diffuses into neighbouring tumour cells that do not express the target antigen. This phenomenon allows not only the direct killing of antigen-positive cells, but also the elimination of adjacent antigen-negative tumour cells, thereby enhancing overall therapeutic efficacy. This effect is particularly beneficial in heterogeneous tumours, where not all cells express the target antigen.
Additionally, the bystander effect is used to disrupt the tumour microenvironment or induce the lysis of infiltrating immune cells that have been rendered inactive by tumour cells. However, this effect must be tightly controlled to prevent damage to healthy neighbouring cells.
Not all ADC payloads exhibit a bystander effect. This property depends on the type of linker (cleavable or non-cleavable) connecting the antibody to the payload, as well as on the physicochemical properties of the payload itself—specifically its ability to cross cell membranes. For instance, an ADC with a non-cleavable linker or a membrane-impermeable payload will not induce a bystander effect, as cytotoxicity remains restricted to cells that have directly internalized the ADC.
CMC considerations for ADCs
Due to their complex structure and mechanism of action, Antibody-Drug Conjugates require specific strategies for their Chemistry, Manufacturing, and Controls (CMC) management. These strategies are essential to ensure the safety, quality, and efficacy of the final product.

Figure 4: Critical Quality Attribute (CQAs) of ADCs from Meng Li, Xueyu Zhao, Chuanfei Yu & Lan Wang, Pharmaceutical Research, 2024.
Safety challenges and quality control
The unique structure of ADCs—combining a monoclonal antibody and a cytotoxic payload via a chemical linker—requires rigorous control of their critical quality attributes (CQAs). Among these attributes, the Drug-to-Antibody Ratio (DAR) is essential for maintaining a balance between efficacy and safety. Advanced analytical methods are employed to detect impurities, monitor stability, and ensure compliance with regulatory standards.
Management of toxic raw materials
The use of highly potent cytotoxic agents in ADC manufacturing requires strict handling and production protocols to protect operators and the environment. The implementation of dedicated containment zones and specialized equipment is crucial to prevent cross-contamination and ensure the integrity of the final product.
Supply chain challenges
The production of ADCs involves a highly complex process requiring seamless coordination between antibody manufacturing, conjugation, formulation, and final fill-and-finish operations. The small batch sizes, combined with stringent requirements for traceability and quality, make cost and timeline optimization particularly challenging. Innovation in manufacturing and logistics processes is vital to meet growing demand while adhering to strict regulatory standards.
In March 2024, the U.S. Food and Drug Administration (FDA) published a guidance document titled Clinical Pharmacology Considerations for Antibody-Drug Conjugates. This document provides key recommendations regarding pharmacological considerations, bioanalytical methods, dosing strategies, and other critical aspects such as immunogenicity and drug–drug interactions (DDIs). These guidelines underscore the importance of robust CMC management to support the development of these innovative therapies.
ADC Market analysis
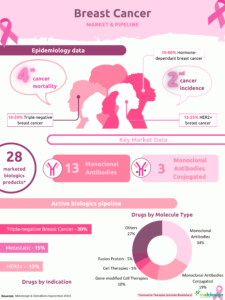
All approved ADCs are currently indicated for use in oncology, targeting either solid tumours (localized) or hematologic (liquid) malignancies. As of 2025, 15 ADCs have been approved globally. Notably, more than 10 of these approvals have occurred over the past six years, highlighting both the significant clinical need for innovative cancer therapies and the growing interest of the pharmaceutical industry in this class of biopharmaceuticals.
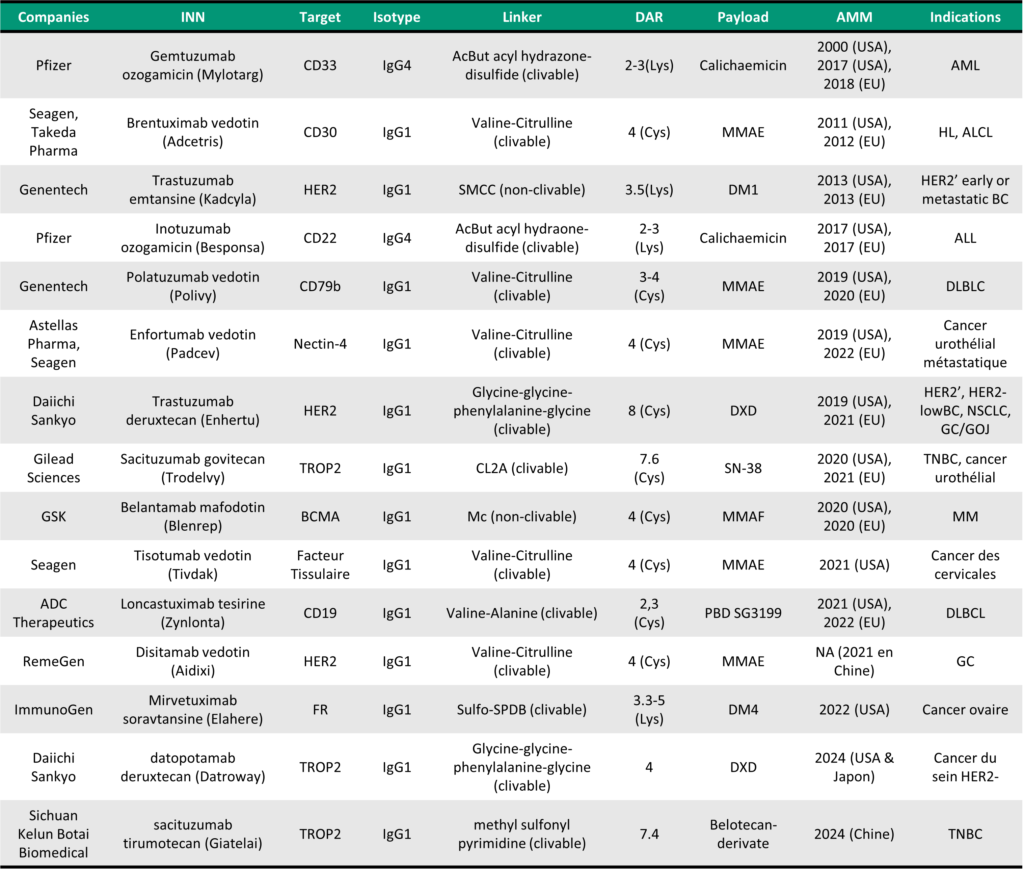
Table 1: Key Characteristics of Clinically Approved ADCs, adapted from Dumontet C, Reichert J, Senter P, Lambert J, Beck A. Nature Reviews Drug Discovery, 2023 and MabDesign analysis.
In 2024, the approval of trastuzumab deruxtecan for the treatment of all solid tumours overexpressing the HER2 receptor marked a major milestone in oncology. This expanded indication extends the use of this targeted therapy beyond breast and gastric cancers, for which it was already approved. The decision was based on clinical data demonstrating the drug’s efficacy across various types of HER2-overexpressing solid tumours, offering new therapeutic options to patients with cancers for which standard treatments are often limited or ineffective.
In clinical practice, this expansion of indications not only provides access to a novel therapeutic option but also enables a more personalized approach to care based on the specific molecular characteristics of a patient’s tumour. This approval highlights the potential of so-called pan-tumour therapies—those capable of targeting a common biomarker shared across multiple cancer types. It paves the way for ADCs to be developed for broader indications and represents a paradigm shift in the strategic development of ADCs.
Market size ADC
The ADC market was estimated to be worth around 7.8 billion dollars in 2023, and is set to grow significantly, reaching almost 44 billion dollars in 2030 according to certain estimates. This growth, estimated at a CAGR of 21%, reflects the growing promise of ADCs as an innovative therapeutic solution in oncology.
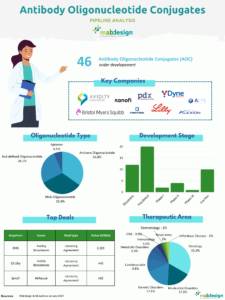
Deals in the field of ADCs also demonstrate the positive dynamics of this market. In 2023, 4 of the 10 biggest deals recorded involved players developing ADCs: Pfizer bought Seagen, an expert in ADCs, for $43 billion; Merck signed a massive partnership agreement with Daiichi Sankyo for $22 billion; Abbvie acquired Immunogen and enhanced its product portfolio for $10 billion; and BMS and SystImmune will collaborate on the development of ADCs for $8 billion.
This momentum continued in 2024, with a number of deals linked to ADCs, including some notable agreements involving Johnson & Johnson, Roche and Merck.
Focus on 2024:

Johnson & Johnson announced the acquisition of Ambrx Biopharma, Inc., a biopharmaceutical company with a technology platform for the design and development of antibody-drug conjugates (ADCs), for a total consideration of approximately $2 billion.

MediLink Therapeutics has announced a collaboration and licensing agreement with Roche for the development of an antibody-drug conjugate (ADC), YL211, which targets mesenchymal epithelial transition factor (c-Met) against solid tumours.

Merck KGaA, has announced a strategic partnership with Caris Discovery, a leading next-generation AI TechBio company to accelerate the discovery and development of antibody-drug conjugates (ADCs) for cancer patients.
Conclusion
The future of ADCs appears highly promising, with ongoing innovations in antibody engineering, cytotoxic payloads, and linker technologies making ADCs increasingly targeted and safer. These advancements pave the way for even more personalized cancer therapies and open new possibilities for applications beyond oncology.

Bispecific ADCs represent a promising new generation of antibody-drug conjugates in oncology, combining the advantages of bispecific antibodies and ADC technology. By simultaneously targeting two tumour antigens or enhancing interactions with immune cells, these bispecific ADCs aim to increase tumour selectivity, reduce resistance mechanisms, and improve therapeutic efficacy.
Several innovative candidates are currently in development across various indications. For instance, some bispecific ADCs simultaneously target HER2 and HER3 to improve treatment outcomes in HER2-positive breast and gastric cancers. Others, such as those directed against CD20 and CD22, are being explored for aggressive B-cell lymphomas, combining targeted cytotoxicity with enhanced conjugate internalization.
Another development strategy involves pairing tumour targeting (e.g., EGFR) with immune cell engagement (e.g., CD3) to activate the immune response in addition to delivering cytotoxic activity. These advances could revolutionize the treatment of solid and hematologic tumours that are resistant to conventional therapies, with multiple clinical trials underway and strong industry interest in these next-generation technologies.
To find out more
- Interested in the key challenges in ADC R&D Explore our training programs: Maîtriser les ADC de la conception à la réussite clinique, Fondamentaux du Quality by Design pour renforcer la R&D de votre entreprise or Evaluation de la développabilité du biomédicament : la CMC, de la molécule au bioprocédé.
- Can’t attend the training but want to learn more about ADC? Download our white paper on ADCs. téléchargez notre livre blanc.
- Want to stay up to date with ADC market trends and major deals? Subscribe to our notre newsletter.
- Looking to connect with key stakeholders in the ADC space? Save the date: 13th Antibody Industrial Symposium (AIS2025), June 25 & 26, 2025, in Tours, France. https://aiscongress.com/agenda/
- Need support for your ADC-related projects—whether in terms of public/private funding or market analysis? Explore our range of services and contact us to discuss your needs. We’d be happy to leverage our expertise to help you reach your goals!
If you have a project involving ADCs, or biomedicines in general, don’t hesitate to take a look at our range of services (analysis, market, financing of innovation, etc.), and to contact us for a chat. We’ll be delighted to put our expertise to work to help you achieve your goals!
Sources
- MabDesign’s expertise & data
- Fu, Z., Li, S., Han, S. et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Sig Transduct Target Ther 7, 93 (2022).
- Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023
- Esnault C, Schrama D, Houben R, Guyétant S, Desgranges A, Martin C, Berthon P, Viaud-Massuard M-C, Touzé A, Kervarrec T, et al. Antibody–Drug Conjugates as an Emerging Therapy in Oncodermatology. Cancers. 2022
- Li M, Zhao X, Yu C, Wang L. Antibody-Drug Conjugate Overview: a State-of-the-art Manufacturing Process and Control Strategy. Pharm Res. 2024
- GobalData, december 2024
- Research & market