MabDesign’s market analyses give you a monthly overview of market dynamics and projects in development for a given indication or technology, to help you better understand the constantly evolving market of biologics and biomanufacturing. This month, an overview of the market and pipeline of therapeutic antibodies.
Market & pipeline analysis
Over the past decades, therapeutic antibodies have progressively established themselves as a major class of biopharmaceutical products. Initially dominated by monoclonal antibodies, the field has expanded to include a growing diversity of formats, reflecting advances in antibody engineering and a deeper understanding of disease biology.
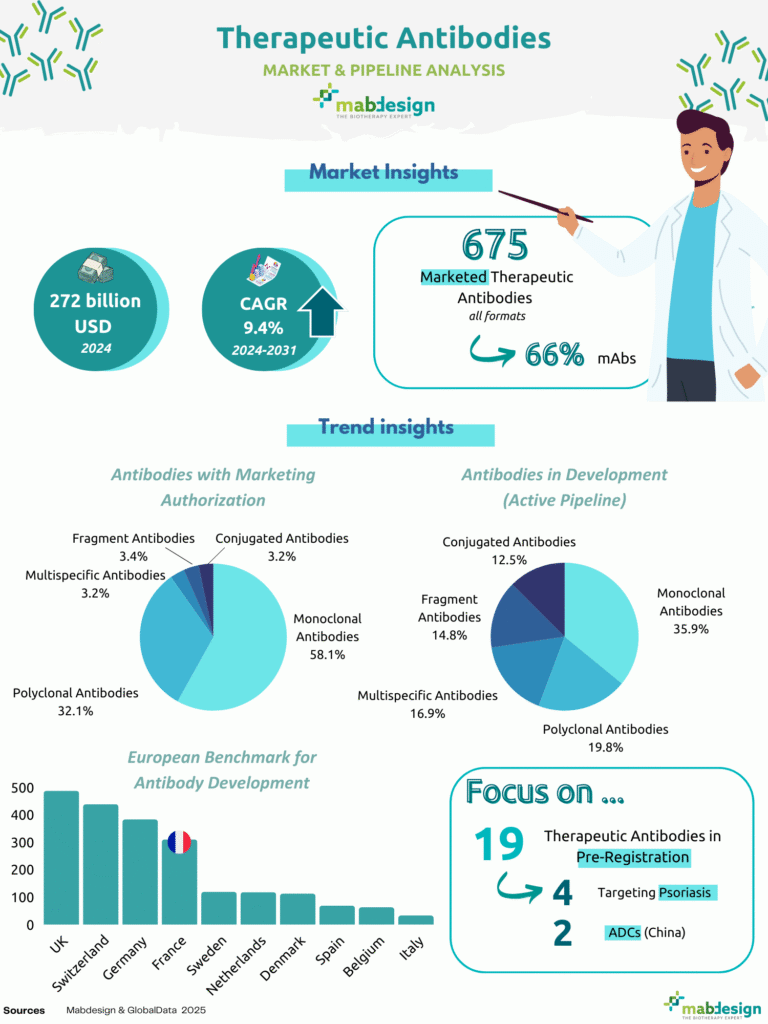
In 2024, the global therapeutic antibody market reached USD 272 billion. Between 2024 and 2031, the market is expected to grow at a compound annual growth rate (CAGR) of 9.4%, confirming the sustained momentum of antibody-based therapies across multiple therapeutic areas.
Key figures
272 billion USD
Market size (2024)
9,4%
CAGR (2024–2031)
675
Marketed therapeutic antibodies :
(all formats)
66%
Share of monoclonal antibodies:
To date, 675 therapeutic antibodies are marketed worldwide, across all antibody formats. Monoclonal antibodies represent the majority of marketed products, accounting for 66% of therapeutic antibodies currently available on the market. This predominance reflects the maturity of monoclonal antibody platforms and their broad clinical use in oncology, immunology and inflammatory diseases.
Current trends
Anticorps commercialisés
Parmi les anticorps thérapeutiques disposant d’une autorisation de mise sur le marché, les anticorps monoclonaux représentent 58,1 % des produits approuvés.
Les anticorps polyclonaux représentent 32,1 %, tandis que les formats plus complexes restent marginaux au stade commercial.
Les fragments d’anticorps (3,4 %), les anticorps multispecifiques (3,2 %) et les anticorps conjugués (3,2 %) ne représentent qu’une part limitée des produits approuvés, illustrant les barrières techniques et réglementaires plus élevées associées à ces formats de nouvelle génération.
675
anticorps thérapeutiques sont commercialisés dans le monde
Marketed antibodies
Among therapeutic antibodies with marketing authorisation, monoclonal antibodies account for 58.1% of approved products. Polyclonal antibodies represent 32.1%, while more complex formats remain marginal at the marketed stage.
Antibody fragments (3.4%), multispecific antibodies (3.2%) and conjugated antibodies (3.2%) together account for a limited share of approved products, illustrating the higher technical and regulatory barriers associated with next-generation antibody formats.
Antibodies in development (active pipeline)
The composition of the active development pipeline highlights a more diversified landscape. While monoclonal antibodies remain the largest category (35.9%), their relative weight is significantly lower than in the marketed portfolio.
Polyclonal antibodies represent 19.8% of candidates in development, followed by multispecific antibodies (16.9%), fragment antibodies (14.8%) and conjugated antibodies (12.5%), including antibody–drug conjugates (ADCs). This distribution reflects ongoing efforts to develop differentiated antibody formats with improved therapeutic profiles.
European benchmark for antibody development
Europe remains a key region for therapeutic antibody development. The United Kingdom, Switzerland and Germany are the leading European countries in terms of antibody development activity, with France positioned among the main contributors.
This European landscape reflects a combination of strong academic research, established industrial capabilities and structured innovation ecosystems supporting antibody development.
Focus on Therapeutic antibodies in late-stage development
- 19 therapeutic antibodies currently in pre-registration
- 4 candidates targeting psoriasis, highlighting the continued importance of immuno-inflammatory indications
- 2 antibody–drug conjugates (ADCs) developed in China, illustrating the growing contribution of Chinese players to late-stage antibody development
Therapeutic antibodies represent a mature yet continuously evolving segment of the biopharmaceutical industry. While monoclonal antibodies continue to dominate the marketed landscape, the development pipeline points toward increasing diversification, with a growing number of multispecific, fragment and conjugated antibody candidates. These trends suggest a progressive evolution of the therapeutic antibody landscape in the years to come.
Download our infographic
projets de thérapies basées sur l’édition du génome (gene-editing therapies) actuellement en développement. (2025)
Want to know more?
MabDesign has acquired and cultivated expertise in all market segments associated with biopharmaceuticals and biomanufacturing. Our team of consultants is available to assist you with all your biotherapy-related projects. Feel free to explore our range of services, including our marketing and strategic analyses, if you would like to learn more!
Sources : MabDesign & GlobalData
Date – Août 2025