Hybridoma technology for the production of monoclonal antibodies (mAbs), awarded the Nobel Prize for Medicine in 1984, revolutionized serotherapy, which previously used patient serum containing mixtures of antibodies. More targeted and therefore more effective, mAbs offer innovative perspectives in the treatment of various diseases, and have become an essential therapeutic strategy in our pharmaceutical arsenal.
Therapeutic strategy and applications
Structure and mechanism of action
A monoclonal antibody is a protein designed to mimic the immune system’s ability to fight different types of aggression. These antibodies are produced in the laboratory and are specifically directed against a single target (antigen), or more precisely against a portion of the target. The target may be a protein, a polysaccharide or a lipid. The antigen may be circulating (free) or expressed on the surface of a cell or even a virus. This binding between antibody and antigen then leads to different modes of action, to provide a therapeutic effect.
The targets of monoclonal antibodies can be varied (cytokines, toxins, cell receptors, etc.). mAbs act on these targets via two main mechanisms: direct or indirect.
Direct mechanisms correspond to the binding of mAb to a ligand or its receptor, which results in the blocking of the interaction between these molecules, and thus the blocking of the cascade of events normally induced following this binding. Examples include monoclonal antibodies targeting the spike surface protein of SARS-CoV-2, which block the interaction of the viral protein with its receptor expressed in particular by cells of the human respiratory system, thus preventing the virus from entering the cells. This direct mechanism of action can also be used with an agonist, to mimic the natural ligand and activate the cascade of signaling pathways.
Indirect mechanisms involve the activation and/or recruitment of immune cells or their components. mAbs can thus activate effector cells, such as Natural Killer (NK) cells, to induce cytotoxicity, or macrophages to induce phagocytosis. Some mAbs can also activate the complement pathway, leading to cytotoxicity.
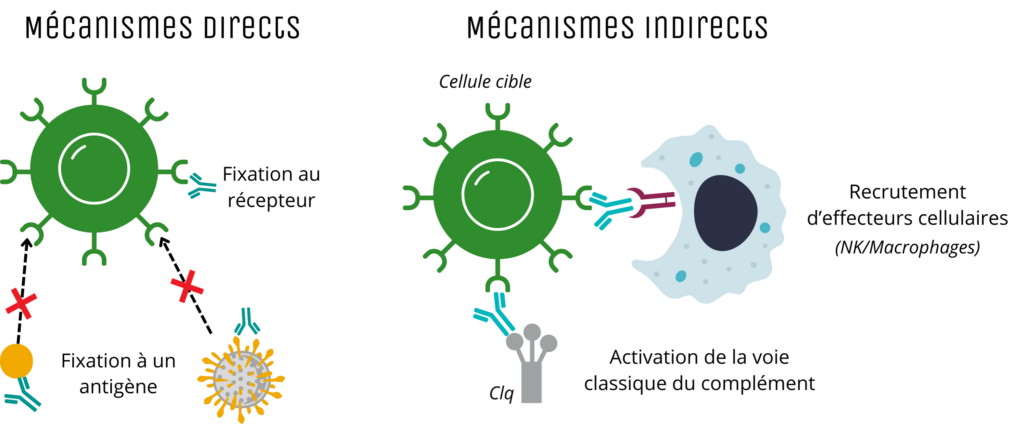
Main mechanisms of action of monoclonal antibodies
Applications and clinical successes
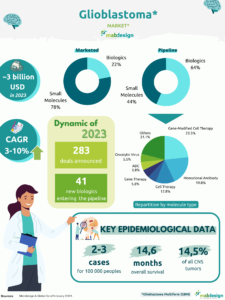
Cancer treatment is one of the fields in which therapeutic monoclonal antibodies have contributed to the most spectacular advances. This success is due in part to the fact that new therapeutic targets have been identified in recent decades, thanks to a better understanding of tumor biology and advances in biotechnology. Monoclonal antibodies offer more targeted treatments than chemotherapy, and have provided therapeutic solutions for breast, colon and ear, nose, and throat (ENT) cancers. These proteins have also demonstrated their interest in lymphoma and leukemia, with the development of anti-CD20 antibodies such as rituximab.

Chronic inflammatory diseases such as rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, psoriasis, Crohn’s disease and multiple sclerosis have in common the involvement of pro-inflammatory cytokines in systemic and articular manifestations. Consequently, these molecules are potential targets for the development of monoclonal antibodies. Rheumatoid arthritis represents the first example of a non-cancerous disease in which a therapeutic antibody has been used. Anti-TNF drugs, such as infliximab, adalimumab and etanercept, are examples of monoclonal antibodies that specifically target TNF-α, a major component of the inflammatory response. Adalimumab, marketed under the brand name Humira by AbbVie, has been one of the world’s top-selling pharmaceutical products for several years.
Although many monoclonal antibodies are being developed to combat cancers and immunological and inflammatory disorders, the emergence of antibiotic resistance and new viruses have also boosted the development of anti-infectious monoclonal antibodies. The Covid-19 pandemic, for example, led to the development of curative treatments using monoclonal antibodies.
Limits and perspectives
Clinical experience shows that the use of monoclonal antibodies is not without toxic effects for patients. Side effects include cytokine release syndrome and the induction of autoimmune pathologies. The immunogenicity of therapeutic antibodies can be a major clinical problem, with the appearance of toxic effects often linked to hypersensitivity phenomena.
Among the limitations associated with monoclonal antibodies, their shelf life in circulation is also a hindrance, and remains an important area for improvement to avoid excessive injection doses.
Despite some technical challenges ahead, the prospects for mAbs are promising and are attracting growing interest in the medical field. On the other hand, an ever finer understanding of molecular biology will enable personalization of monoclonal antibody monoclonal antibody treatments in the future.
Progress is also being made in the development of new monoclonal antibody formats, such as bispecific antibodies that can bind to two different targets, or antibody fragments that can go deeper into solid tumors. These innovative formats broaden therapeutic possibilities. The number of clinical applications is set to increase in the future, particularly in neurodegenerative and cardiovascular diseases. In addition, low-cost biosimilar monoclonal antibodies can also meet the needs of developing countries.
Overview of the monoclonal antibody market
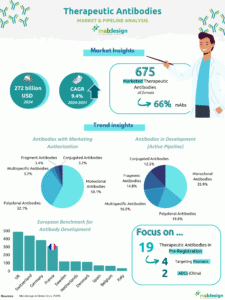
Monoclonal antibodies represent one of the largest categories of marketed biotherapies in terms of number of products, alongside therapeutic proteins, peptides and biopolymers. There are 168 marketed products (excluding biosimilars) worldwide and 5,360 products in development.
Growing interest in the field is reflected in the number of agreements (mergers, acquisitions, partnerships, etc.) between players. In the space of almost 10 years (2013-2023), the number of agreements has risen by 29%, from 67,471 in 2013 to 86,927 in 2023. Geographically, the market is dominated by the United States, which accounts for 95% of the number of agreements, compared with 2% for Europe.
In 10 years, the field of monoclonal antibodies has evolved spectacularly, with the number of antibodies and derivatives on the market tripling(2009-2019).
On a national level, this growth has been supported and structured: the Programme Investissement Avenir (PIA) has strengthened both public and industrial activities, with the creation of the MabImprove Laboratory of Excellence, the Mi-Mabs industrial demonstrator, and more recently, industrial integrators as part of the “Grand Défi Biomédicaments” and the PEPR BBTI and its ACCREDIA project. The “Stratégie d’Accélération des Biothérapies et de la Bioproduction” (Biotherapies and Biomanufacturing Acceleration Strategy), launched in 2022, has also supported antibody-related research and development projects, with various prizewinners working in this area. This structuring also enabled MabDesign to emerge and position its initial expertise in immunotherapy, before moving into the broader biomedical sector.
Today, mAbs represent an immense therapeutic hope for many diseases, and a flourishing, constantly evolving market.
If you have a project involving mAbs, or more broadly biotherapies or biomanufacturing, take a look at our range of servicesandcontact us to exchange. We’d be delighted to use our expertise to help you achieve your goals !
Take a look at our training courses on the subject to stay up to date on the field, or our Antibody Industrial Symposium to keep abreast of the latest innovations.
Sources
- MabDesign expertise and data
- GlobalData February 2024
- Soigner avec les anticorps monoclonaux, CEA
- Anticorps monoclonaux en thérapeutiques, éditions 2009 et 2019, Médecine/Sciences
- Antibody Industrial Symposium, MabDesign & LabEx MabImprove
Market studies manager Biotechnology engineer