Each month, MabDesign’s market analyses give you an overview of market dynamics and projects in development for a given indication or technology, to help you better understand the constantly evolving market for biomedicines and biomanufacturing. This month, an overview of antibody-oligonucleotide conjugate (AOC) projects currently in development.
Genome editing refers to all the techniques used to introduce, modify or delete specific DNA sequences within a living organism. Unlike traditional gene therapy approaches, which involve inserting a functional gene into a cell without modifying the existing DNA, genome editing offers an unprecedented level of precision.
This approach makes it possible to directly correct the mutations responsible for genetic diseases, reprogram immune cells to fight cancer, or improve certain characteristics of living organisms in biotechnology and other fields.
Of all the gene-editing technologies, CRISPR-Cas9 is the most widely publicised and revolutionary. Derived from a natural bacterial defence mechanism, it enables a precise DNA sequence to be targeted and modified using an enzyme (Cas9) guided by RNA. Its ease of use, speed and effectiveness make it a tool of choice for research and clinical applications.
Other technologies have also been developed to modify the genome:
- TALEN (Transcription Activator-Like Effector Nucleases): these artificial nucleases enable targeted modifications but are more complex to design than CRISPR.
- ZFN (Zinc Finger Nucleases): one of the first genome-editing systems used in gene therapy, although more difficult to programme than CRISPR.
- Advanced CRISPR-based nucleases (CRISPR-Cas12, CRISPR-Cas13, etc.): variants of CRISPR offering new possibilities, such as RNA modification or more precise modifications without cutting DNA.
MARKET
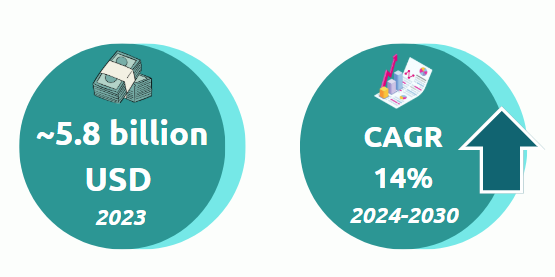
The market for therapies based on genome editing has been valued at $5.8 billion in 2023 and is forecast to grow strongly, with a CAGR of 14% to 2030.
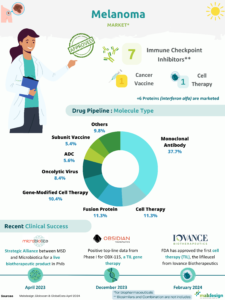
The first CRISPR-based drug, Casgevy, was recently approved to treat sickle cell anaemia and beta-thalassaemia. This therapy is based on editing the patient’s haematopoietic stem cells to correct the underlying genetic abnormality.
Therapeutic Areas
PIPELINE
Other candidates are in clinical and non-clinical development, notably for :
- Rare diseases (amyloidosis, muscular dystrophy, etc.)
- Cancers, with CAR-T cell editing to boost immunotherapy
- Infectious diseases, such as the exploration of CRISPR against HIV
The rise of biotechnology and investment in genome editing have accelerated research in this field, with several start-ups and major laboratories racing to develop the next generation of therapies.
Development phases and therapeutic areas
Gene-edited Therapies
under development*
Delivery system**
No Data Found
*For active pipeline
**For disclosed systems
Gene Editing Technology
The success of therapies based on genome editing relies to a large extent on the delivery methods used to bring the editing tools (such as CRISPR-Cas9, TALEN or ZFN) to the target cells. Two main categories of vector are used:
- Viral vectors, which account for 60% of the technologies disclosed for products in development,
- Non-viral vectors (LNP, nanoparticles, plasmids, transposons, etc.), which account for 40%.
Want to know more ?
To meet with industry and academic experts in gene and cell therapies and discover the latest innovations in the sector, don’t miss the 2nd edition of our Innovation for Cell and Gene therapies (ICGT) Congress, on May 22 and 23, 2025 on the Sanofi Gentilly Campus! You can register now, or contact us for more information.
MabDesign has acquired and cultivated expertise in all market segments associated with biomedicines and biomanufacturing. Our team of consultants is available to support you in all your projects related to the biotherapeutics sector. Please feel free to explore our range of services, including our marketing and strategic analyses, if you’d like to find out more!
Sources : MabDesign & GlobalData
Date – March 2025